Quantum Chemistry: Understanding the Dance of Atoms and Electrons
Introduction
Imagine trying to understand how atoms really behave, not just as tiny balls bouncing around but as systems of energy and waves. This is what quantum chemistry enables us to do. Imagine having a super-microscope that lets us see electrons dance around atoms and really form molecules.
What is Quantum Chemistry?

Quantum chemistry is the union of the strange, weird rules of quantum mechanics and the study of chemical systems. It allows us to know:
- How the electrons behave in atoms and molecules
- Why some chemicals react and others do not
- How light behaves when it interacts with matter
- What gives materials unique properties
Key Concepts
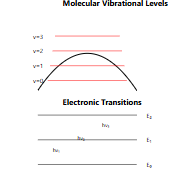
1. Wave-Particle Duality
- Electrons, as well as all matter, can behave as either a particle or a wave
- This is why electrons form “clouds” around atoms, rather than orbiting like planets
- The mathematical description of these waves helps predict chemical behavior
2. Quantum Numbers
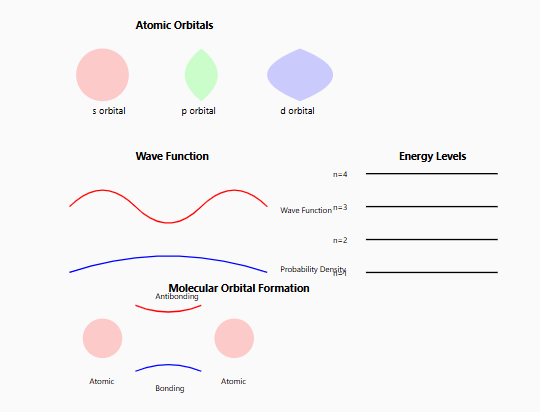
Four special numbers describe each electron in an atom:
- Principal Quantum Number (n)
- Determines the main energy level
- Like floors in a building
- Starts at 1 and can go up indefinitely

- Angular Momentum Quantum Number (l)
- Describes the subshell shape
- Like rooms on each floor
- Values from 0 to (n-1)
- Gives us s, p, d, and f orbitals
- Magnetic Quantum Number (ml)
- Shows orbital orientation in space
- Like different windows in each room
- Ranges from -l to +l
- Spin Quantum Number (ms)
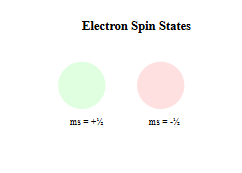
- Accounts for electron’s intrinsic spin
- Can only be +½ or -½
- Like spinning clockwise or counterclockwise
3. Atomic Orbitals
Different kinds of electron clouds:
- s-orbitals: Spherical shapes
- p-orbitals: Dumbbell shapes
- d-orbitals: More complex patterns
- f-orbitals: Even more intricate shapes
4. Molecular Orbital Theory
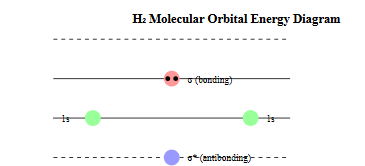
- Indicates how atomic orbitals combine to create molecular orbitals
- Describes chemical bonding at the quantum level
- Provides predictions of molecular properties and reactivity
Real-Life Applications

1. Drug Design
- Predicting how molecules will react with proteins
- Producing more potent medicines
- Understanding drug side effects
2. Materials Science
- Production of new semiconductors
- Improved solar cells
- Storing material with higher strength
3. Spectroscopy
- Chemical compositions determination
- Star composition analysis
- Detection of substance in medical tests
4. Catalysis
- Industrial process enhancement
- Green chemistry method improvement
- Energy system improvement
Contemporary Areas of Research
1. Chemistry by Quantum Computation
- Simulation of complex molecular systems
- Solutions to hitherto unsolvable chemical problems
- Prediction of chemical reactions
2. Quantum Biology
- Explaination of photosynthesis
- Explanation of the Bird navigation
- Elucidation of enzyme catalytic processes
3. Advanced Materials
- Designing quantum dots
- Generation of novel nanomaterials
- Developing “smart” materials
Tools and Techniques
1. Computational Method
- Density Functional Theory (DFT)
- Ab initio calculation
- Molecular dynamics simulations
2. Experimental Techniques
- Nuclear Magnetic Resonance (NMR)
- Electron Microscopy
- Laser Spectroscopy
Future Directions
1. Emerging Technologies
- Quantum sensors
- Molecular machines
- Smart catalysts
2. Unsolved Problems
- High-temperature superconductivity
- Efficient solar energy conversion
- Complex protein folding
How to Get Started
For Students
- Basic Prerequisites
- Strong foundation in basic chemistry
- Understanding of waves and energy
- Basic calculus concepts
- Learning Resources
- Online courses in quantum chemistry
- Interactive molecular modeling software
- Chemistry simulation games
Practical Exercises
- Simple Calculations
- Electron configurations
- Energy level diagrams
- Simple wave functions
- Computer Modeling
- Use of molecular viewers
- Basic quantum chemistry software
- Online simulation tools
Quantum chemistry is a fascinating world where the boundaries between physics and chemistry blur. It helps us understand the fundamental nature of matter and chemical reactions, which leads to breakthrough discoveries in medicine, technology, and environmental science.
Note: This guide provides an overview of quantum chemistry concepts. The field is constantly evolving with new discoveries and applications.
